Trust helps to develop life-saving technology for trauma patients
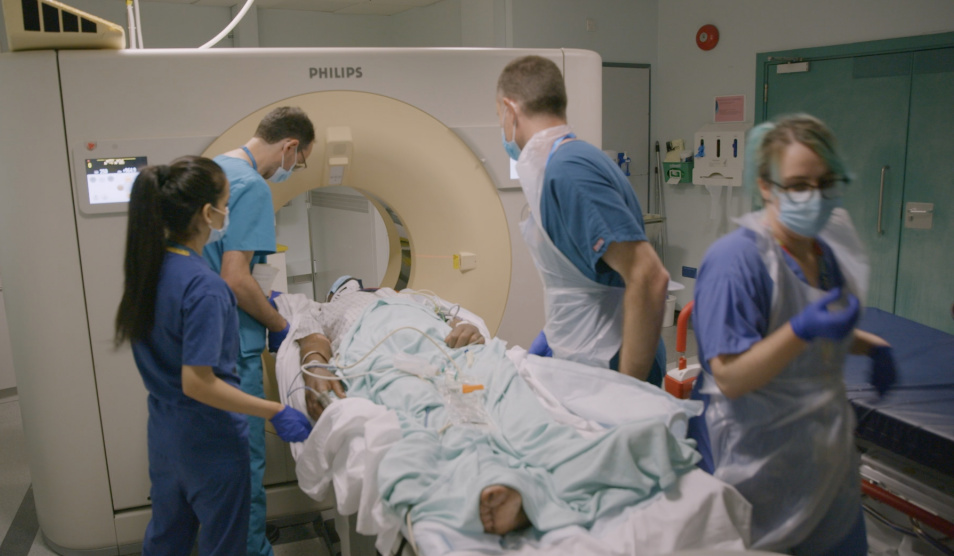
Imperial College Healthcare NHS Trust is helping to develop new technology to quickly detect whether a patient is suffering from internal bleeding, potentially saving the lives of trauma patients and those with severe bleeding.
Specialist trauma surgeons from St Mary’s Hospital, the National Institute of Health Research Diagnostic Evidence Cooperative (NIHR DEC), and instrumentation designers from developers Highland Biosciences (HLB), are in the process of trialling the hand-held device called CoaguScan.
The device, which is in the early prototype stage and is yet to be tested directly on patients, helps clinicians determine whether a patient is suffering from internal bleeding and requires a blood transfusion by analysing the blood in situ for signs of coagulopathy. Coagulopathy occurs when a patient’s blood has lost the ability to clot putting them at risk of massive blood loss. Patients with coagulopathy require a blood transfusion, but this occurs in less than one-third of trauma cases.
Currently, it can take up to one hour to determine whether a patient requires a blood transfusion. As a result, blood transfusions are administered to all patients who arrive with trauma and are bleeding uncontrollably, as a precaution. This equates to more than 8000 patients per year in England alone, meaning vital blood supplies are often given to patients who do not benefit from them.
Giving unnecessary blood transfusions can also be detrimental to the patient if the body reacts badly to the transfused blood, potentially leading to health complications and a lengthier hospital stay.
The CoaguScan device is also designed to help clinicians to determine the exact number of blood products a patient requires from their blood transfusion. This helps to ensure that they do not ‘bleed out’ blood they have just received.
St Mary’s Hospital is one of four major trauma centres in the London, covering central and north west London. Each year is sees around more than 3000 trauma patients. It is the first Trust to trial the new device.
Mansoor Khan, consultant military and trauma surgeon with the Trust said:
“This device has potential to transform how we look after trauma patients by enabling us to know extremely quickly whether a blood transfusion is needed and the make-up of that blood. This means we can provide care that is better tailored to the individual rather than adopting a one size fits all approach.
“The first ‘proof of concept’ phase trial at St Mary’s Hospital showed promising results and we are confident this device has potential to help us save more lives in the future.”
The CoaguScan is small and robust which means it can follow the patient from major trauma into surgery and onto the ICU to the ward, giving results when they are needed after each therapeutic intervention in a few minutes compared to the delays of up to an hour experienced currently.
Dr Richard Day, Managing Director Highland Biosciences (HLB) who are developing the CoaguScan said:
“The results of the trial so far are really promising and we are thrilled to be partnering with Imperial College Healthcare NHS Trust on this world first pilot.
“By partnering with the Trust, we have been able to perform a practical demonstration of the technology we have developed. This has shown the potential for it to be deployed wherever and whenever the transfusion of blood products is used as a treatment to stop uncontrolled bleeding.
“Given St Mary’s reputation as one of the busiest trauma centres in the UK with very good outcomes for patients, it was ideal location for us to test CoaguScan in the first proof of concept phase.
“We are now hoping to secure funding to roll out the next stage of the project which will see CoaguScan being tested in major trauma centres across the UK.”
Emily Ashworth, Senior Divisional Research Nurse with the Trust said:
“Our team are very proud and excited to be working on such a pioneering piece of equipment and can see how this technology has potential to change the way in which we treat rapidly bleeding trauma patients in the future.”
The proof of concept phase trial involved using the CoaguScan to test blood which has already been analysed using traditional methods and comparing the results.
The next phase of the project will involve:
- Multi-centre trials of prototype instruments with other centres of excellence.
- Further collaboration with the Trust and the NIHR DEC London to study the impact on patient outcomes when this technology is used during trauma and identifying improvements in efficiencies and the economic benefits to the NHS if the technology is adopted.
- A nationwide consultation with clinicians to help refine the technology and develop the concept further based on the feedback received.